Navigating the FDA takes experience. Fang can help.
How can we help?
The IVD market in the US is valued between $40 – $83.3 billion and is growing rapidly with pandemics and an increasingly older population driving demand.
Whether your product is a reagent, instrument or software, the Fang Consulting team has a solid understanding of in vitro diagnostic devices and can assist your team prepare for a submission.
US Services
- Establishing General Controls
- Meeting CLIA standards
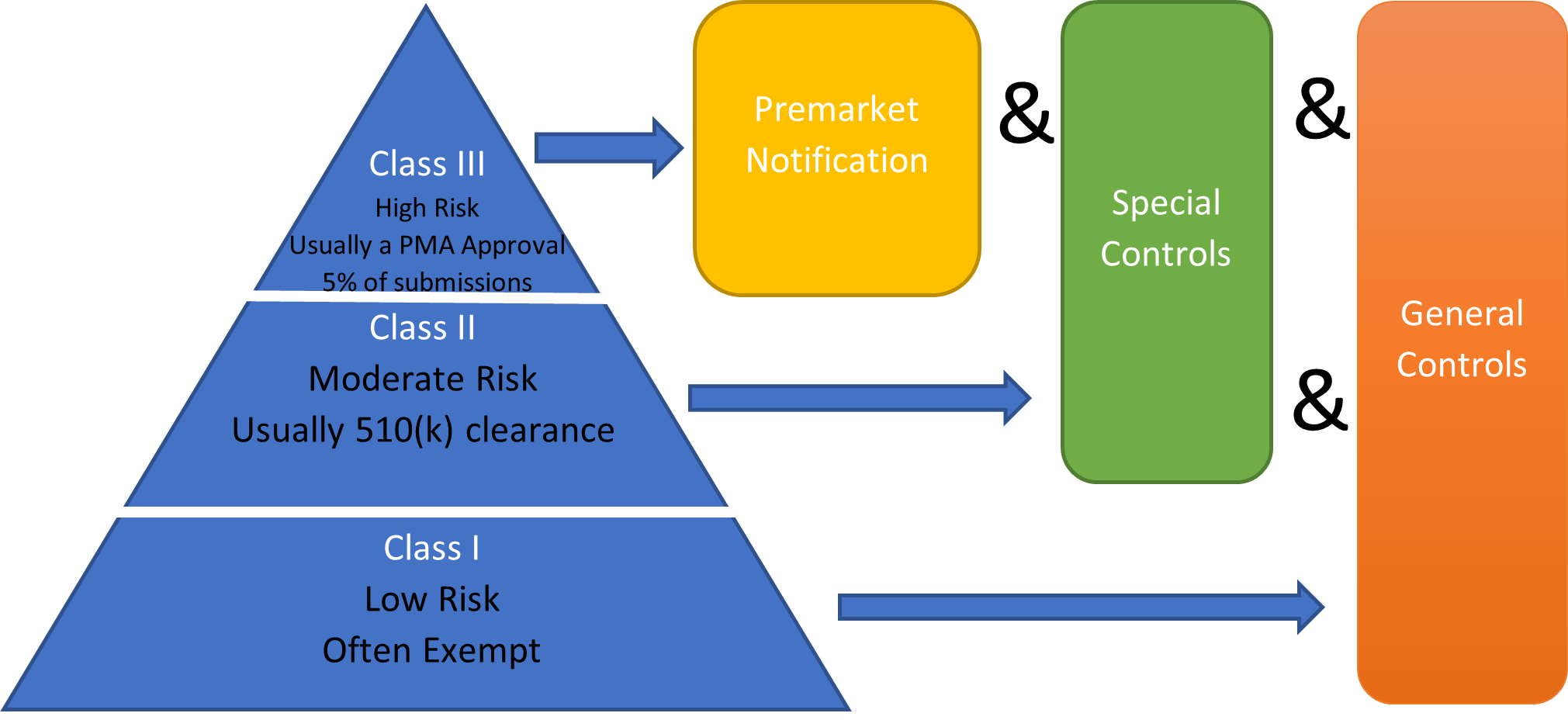
- 510(k) Submissions
- Classification review
- General Purpose Reagent (GSP) determination and classification
- Analyte Specific Reagent (ASR) determination and classification
- Type of 510(k) needed: Traditional, Special or Abbreviated
- De Novo submissions
- Q-Submission meetings
- Pre-submissions (Pre-subs)
- Testing review
- Predicate or reference method review
- New device review
- Submission Issue Requests (SIRs)
- Feedback for specific questions
- PowerPoint presentation
- Team rehearsals
- Pre-submissions (Pre-subs)
- Labeling review
- Establishment Registration & Device Listing
- Current Good Manufacturing Practices (cGMPs) and Quality System (QS) questions
- Medical Device Reporting (MDR)






